- Evolution of catalytic converters
- It is preferable to install a universal catalyst:
- Get rid of soot!
Catalysts appeared just in the first half of the 70s and, as you know, US automakers were at the forefront here for a while. Curiously, in addition to directly reducing the toxicity of exhaust, these catalysts have dragged along with the modernization of several areas of development of the automotive industry. This was due to the very principle of their action, which, by the way, has not changed until now.
For some time, and on different models, catalysts coexisted with carburetors or mechanical injection. For example, Chrysler, having burned itself with electronic fuel supply back in the 50s, was in no hurry to transfer its engines to it. But limiting norms for the content of various elements in the exhaust were approved by law. It was necessary to somehow link the catalyst and the carburetor. Ecology is the engine of progress.
Evolution of catalytic converters
The metal barrel, located between the exhaust manifold and muffler-resonators, has a longitudinal honeycomb inside, where the surface is covered with a layer of a special substance, which is a catalyst. Let's just say that catalyst platinum with the addition of palladium is used as the latter, which converts harmful CO and CH into carbon dioxide and water. Such catalysts were called two-component, that is, capable of neutralizing only a couple of toxic components. In 1977, rhodium was added, so that nitrogen oxide was transformed into a mono-element. Catalysts have become three-component. So, this relatively simple chemical process proceeds without problems only under ideal laboratory conditions. In real operation, manufacturers are faced with the fact that the correct operation of catalyst and, in general, its resource is under constant threat. As it turned out, the catalyst can properly work only when the ratio of the combustible mixture by air and fuel is in the proportion of 14.5–14.7: 1. Deviations to one side or the other reduce the conversion efficiency of CO and CH or NO.
The catalyst honeycomb structure with ultra-small cross-section tubes was chosen because this way it is possible to maximize the area on which the substance acting as a catalyst is applied. Accordingly, the volume of catalyst "processing" of exhaust gases is also growing.
To make the air-fuel mixture stable, carburetors began to be supplemented with electronic control. In 1975, in the United States, transistor ignition systems appeared, which minimized gaps in sparking, from which fuel burned out in catalyst. We turned to the exhaust gas recirculation system, which, by lowering the combustion temperature of fuel mixture, reduces the number of nitrogen oxides. Finally, the fight for the environment, as well as the desire to remove more power, also contributed to the early introduction of electronic injection, a system that can fully unleash the potential of catalysts. At the same time, in the 70s, another event took place, under the influence of laws and public, oil producers abandoned additives based on tetraethyl lead.
And catalysts continued to be improved. A quarter of a century ago, a keg moved from under the underside of a car into the engine compartment, right next to the exhaust manifold. This was necessary for the catalyst fastest-warming up and reducing harmful emissions immediately after starting the car. The catalyst substances begin to act only at 250-300 degrees. Later, the development of separate electric heating with a capacity of up to several kW was proposed. There were systems of two catalysts, where the first was located directly in the path and worked while the main unit was warming up. Adsorption traps for hydrocarbons were set up, holding them until the catalyst reached the operating temperature. Catalyst experiments have been and are being conducted with filler materials. Refractory ceramics are relatively heavy and far from ideal for creating ultra-thin catalyst honeycombs. The metal for cells was used before, and now it is being used again, at a different technological level, using various bimetallic alloys. Lightweight, resistant to temperature, thin as foil, so you can significantly increase deposition area of platinum, palladium, and rhodium.
Car owners try new options installing a universal catalyst. But universal catalyst, like the original, is highly dependent on external factors. It is impossible to give a full guarantee on it. Usually, they serve 60-80 t.km, but they can fail earlier if there is a failure in an ignition system or an engine operating system.
It is preferable to install a universal catalyst:
-
If the car is under 5 years old;
-
If you go through the inspection yourself;
-
If you don't want to pollute the atmosphere;
-
If you are planning to travel abroad.
Limiting parameters of toxicity of exhaust gases are regulated by law in many countries by special organizations. For example, in the United States, this issue is controlled by the Department of Environmental Protection, and California has its department for overseeing air resources (California Air Resources Board). In Japan, the Ministry of Transport deals with the purity of the exhaust, in Europe it is a special European Commission. As a rule, the degree of environmental concern directly depends on the economic and social level of the country's development. Also, norms regulated by the law must be strictly implemented in practice.
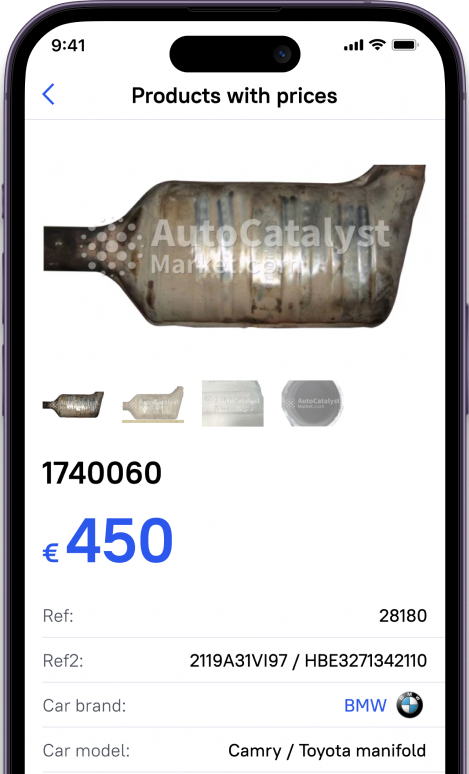
That is why many catalyst inventions had been implemented into the car item, responsible for cleaning the exhaust gas catalyst is usually combined with an exhaust manifold. Such an arrangement of a former affects its efficiency, but makes assembly more expensive and can lead to damage to a piston group.
The lambda sensor, which determines the percentage of oxygen in the exhaust, and sends a signal to correct the mixture, appeared back in 1976. Then a sensor was added behind the catalyst, which monitors the quality of gas cleaning.
Bosch started measuring oxygen content back in 1968, but the first lambda sensor did not appear until eight years later - on the Volvo 240/260 for the North American market. A little later, other manufacturers also realized that without feedback from the control unit, the catalyst cannot provide maximum efficiency.
Get rid of soot!
In the early 2000s, it was the turn of diesel engines. They, equipped with already familiar catalysts, began to be equipped with diesel particulate filters. The fact is that the temperature of exhaust gases in no-load modes is lower here than in gasoline engines. It is not enough for complete combustion of carbon, so particulate matter or soot is obtained, which can pass through the catalyst.
The particulate filter is located in front of the catalyst. It also has platinum and the same honeycomb channels. Only the latter are staggered and are divided into inlet and outlet. And between them, there are partition catalyst filters that contain solid particles with nitrogen oxides. It seems to be the same filter, but with a fundamental difference. It does not accumulate solid particles. Catalyst filter burns them out with the help of oxygen released from nitrogen oxides and an additional nozzle that supplies diesel fuel to the unit.
This complicated but small automotive part plays a huge role in cleaning the environment. Take care of your catalyst!